Pilot Scale Molecular Distillation
2)Supply Single stage, Dual stage, Three stage.
3)High vacuum degree: 0.1Pa.
4)Control system: ABB.
5)Material:316 stainless steel.
6)OEM & ODM support.
7)Warranty:1 Year.
8) Support 24h continuously working
- Product Description
GMP-Certified Pilot Scale Molecular Distillation Systems for Precision Separation
Engineered for R&D, Process Scaling, and Heat-Sensitive Material Purification
Whether you’re refining APIs, purifying nutraceuticals, or developing novel polymers, our Pilot Scale Molecular Distillation systems deliver unmatched precision. With ISO 9001, CE, and GMP certifications, these modular units are trusted by leading pharmaceutical, chemical, and cosmetic innovators globally.
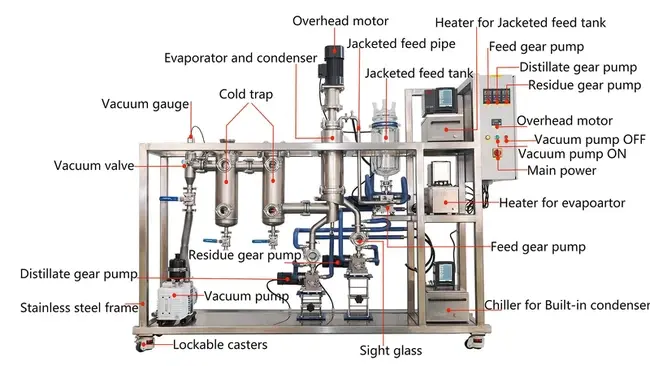
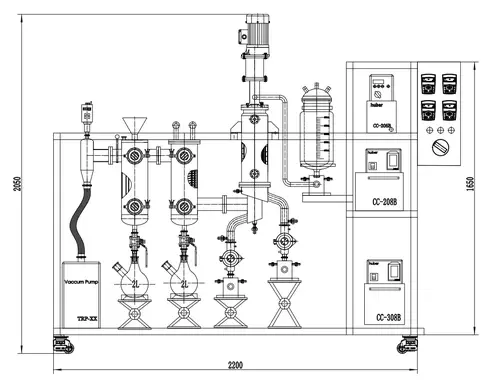
Why Choose Our Pilot-Scale Distillation Technology?
✅ GMP/ISO Compliance
Globally validated systems with FDA 21 CFR Part 11-ready controls for pharma and nutraceutical production.
✅ Modular Scalability
Transition seamlessly from pilot batches (1-100L/hr) to full-scale processes with interchangeable condensers.
✅ Low-Temp Efficiency
Operate at <1mbar vacuum with 40°C–200°C thermal range to preserve bioactive compounds like CBD or omega-3s.
✅ Material Versatility
Choose 316L stainless steel, glass-lined, or Hastelloy contact surfaces for corrosive or ultra-pure applications.
Industry-Tailored Solutions
Pharmaceuticals
- Purify heat-sensitive APIs with ≤0.5% degradation
- Integrated CIP/SIP cycles and audit trails for FDA compliance
Nutraceuticals
- Retain 98%+ active compounds (e.g., vitamins, marine lipids)
- NSF-certified designs for global food safety standards
Specialty Chemicals
- Process viscosities up to 10,000 cP with anti-corrosion decks
- ATEX-compliant configurations for solvent recovery
Cosmetics
- Preserve terpene profiles in essential oils with <2-second residence time
- Ra ≤0.4μm cosmetic-grade finishes
Research Institutes
- Adjustable throughput (0.01–100 kg/hr) for multi-disciplinary projects
- Open-architecture controls for real-time mass spectrometry integration
Technical Excellence in Every Component
Short Path Distillation
Minimize thermal exposure with a <2-second material transit time.
Multi-Stage Condensers
Achieve 99.8% purity in single-pass operations for high-value extracts.
IoT-Ready Monitoring
Track viscosity, temperature, and pressure via ABB PLCs with GMP audit data exports.
Trusted by Industry Leaders
Case Study: Reduced API purification costs by 30% for a Top 10 Pharma Company using our low-temp Pilot Scale Molecular Distillation system.
Certifications: ISO 9001 | CE | ASME BPE | NSF
Surface Finish & Vacuum Degree

Factory Strength

Exhibition

Packaging & Transportation


FAQ
What GMP features ensure API purity?
Our systems include validated CIP/SIP cycles, ±0.5°C temperature control, and 21 CFR Part 11-compliant data logging.
How is thermal degradation prevented?
Short-path geometry and high vacuum (0.1Pa) reduce heat exposure, critical for omega-3s or fragrances.
Can I reconfigure the system for different chemicals?
Yes – modular evaporator decks and flange-compatible designs allow quick adaptations.
What certifications apply to nutraceutical exports?
We provide EU Cosmos, NSF, and Halal/Kosher options with full material traceability.
Contact Us
Ready to Optimize Your Purification Process?
Contact Us Today: Email info@welloneupe.com for Pilot Scale Molecular Distillation personalized consultation.








